Why is Berkeley Pit water acidic?
Underground mining in Butte left a network of tunnels and shafts that contained exposed rock. Much of this exposed rock contains significant amounts of iron sulfides (e.g., iron pyrite or “fools gold”). In a naturally occurring bedrock system, iron pyrite remains out of contact with oxygen in the air. However, when iron pyrite is exposed to oxygen and water it creates sulfuric acid.
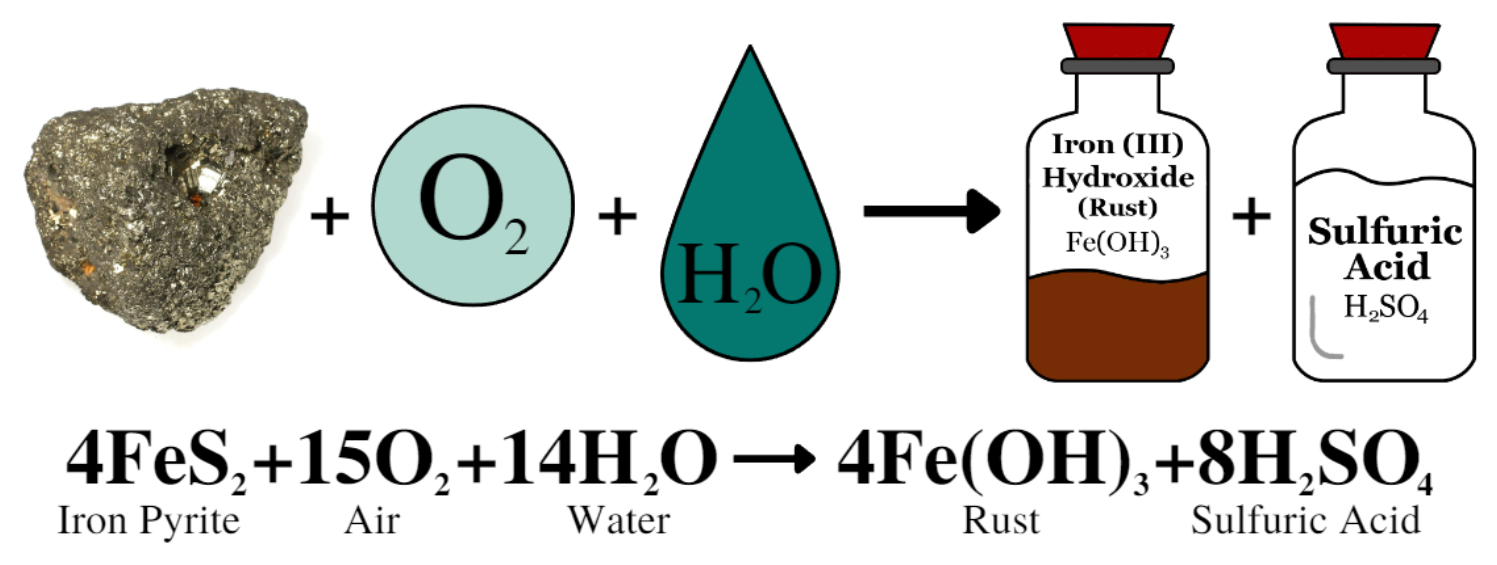
In the case of the Butte Mines, nearly 10,000 miles of tunnels were left exposed to air for decades. When mining in the Berkeley Pit stopped, the underground dewatering pumps were shut off and groundwater returned. The returning groundwater interacted with the air-exposed iron pyrite creating groundwater contaminated with sulfuric acid. The sulfuric acid generated helped to break down other metals and minerals found in the bedrock in the Butte Hill, adding even more contamination to the water. The network of underground mines is directly connected to the Berkeley Pit, meaning that the contaminated water from the mines is now filling the Berkeley Pit.
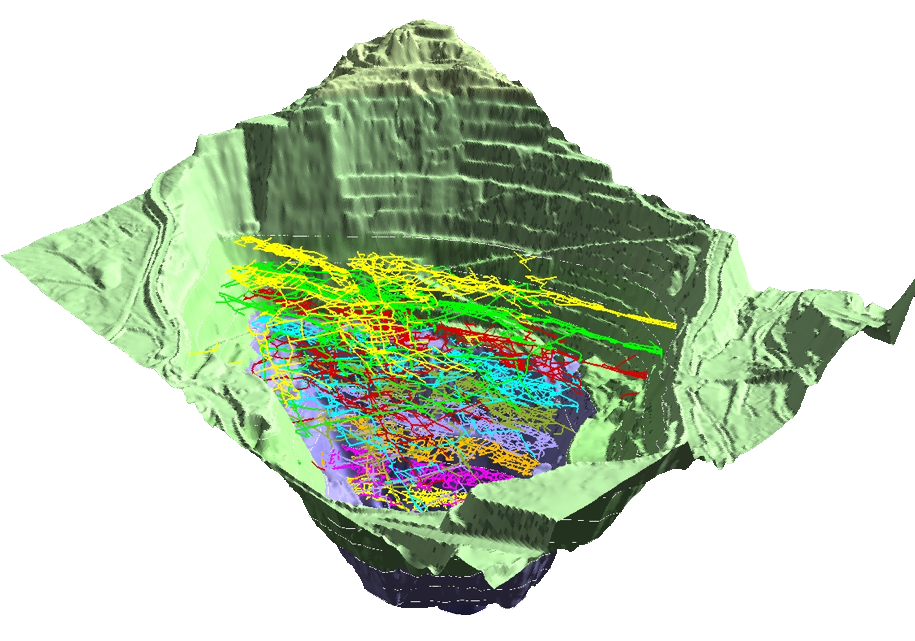
This 3-D rendering from Montana Bureau of Mines and Geology (MBMG) shows where underground tunnels existed in the area that became the Berkeley Pit. While the tunnels shown here no longer exist because they were excavated by the Berkeley Pit operations, the image illustrates the interconnectedness of the Berkeley Pit and the network of historic underground mining tunnels.
How did metals and minerals get in the Berkeley Pit water?
The Berkeley Pit water contains contaminants that are naturally occurring in the ore bodies in Butte. Underground mining exposed the ore bodies containing these metals. Returning groundwater interacts with these exposed metals and minerals and dissolves into the water. The acidity of the water results in additional breakdown of metals and minerals thus increasing the concentration of metals and minerals in the water collecting in the Berkeley Pit.
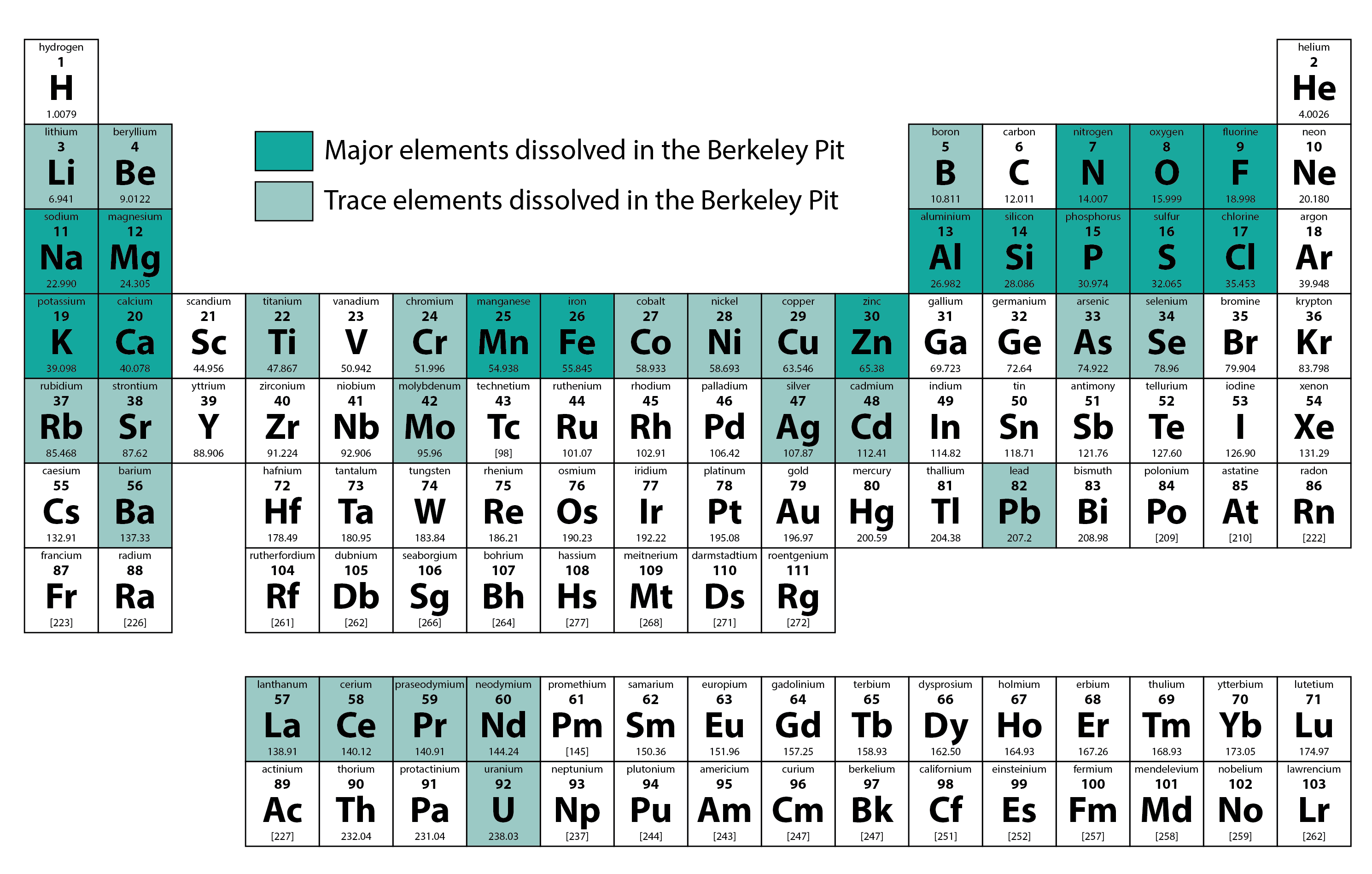
Examples of elements found in the Berkeley Pit, including those found in compound forms. Major solutes (dissolved elements in the water) include fluoride, sodium, magnesium, aluminum, sulfate, chloride, potassium, cadmium, and manganese.
Is the water harmful to life?
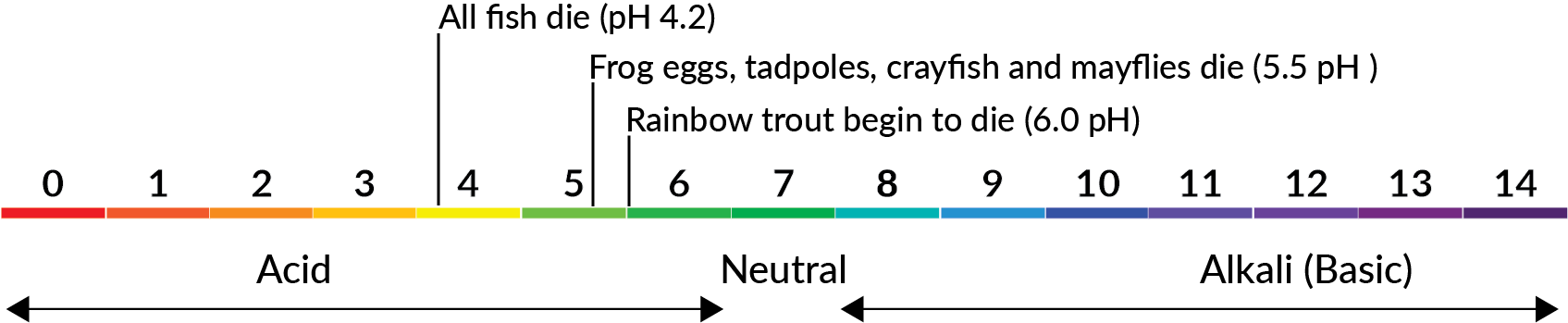
The water in the Berkeley Pit has a low pH; pH is a measure of the acidity or basicity of a liquid. Water in the Berkeley Pit has a pH that has historically measured around 2.5. Over time, treatment has improved the Berkeley Pit’s pH to measure at or above 4.0. The scale below illustrates the pH of familiar materials compared to Berkeley Pit water.

Acidic or low pH is too acidic to support aquatic life like fish or small macroinvertebrates. Drinking water has a pH value of 7.0, the Berkeley Pit has a pH that has ranged between 2.5-4.5. The graphic below illustrates the pH levels associated with survival of aquatic life and acidic pH threatens fish and other aquatic species.
In addition to the Berkeley Pit’s acidity, the high concentrations of metals in the Berkeley Pit water are not suitable for human or aquatic life. The table below shows the copper concentration (in parts per billion) found in the Berkeley Pit vs the aquatic life and human health standard.

Can anything live in the Berkeley Pit?
Yes, researchers have discovered extremophilic organisms surviving in the Berkeley Pit. Extremophiles are organisms that can live in extreme conditions such as temperature, acidity, alkalinity, or chemical concentrations likely inhospitable to other living things. Researchers have identified many different species of microorganisms in the Berkeley Pit and research continues to understand these communities.
Please contact PitWatch if you’re interested in more information about extremophiles and we will connect you with appropriate bibliographic information.
